Are you ensuring your SOPs have been validated according to GPP & GMP?
The Cannabis Act: GPP and GMP for Cannabis Quality Assurance
The Canadian Cannabis Act came into effect on October 17, 2018 in order to prevent underage
cannabis use and disrupt the cannabis black market. Due to this, only a licensed cannabis producer is able to have a share in the market. The Cannabis Act (or Bill C-45) created a framework for controlling the production, distribution, and sale of cannabis for recreational and medical purposes.
The Cannabis Act penalizes sale to underaged youth, and prohibits producers from using packaging or creating products that are marketed to underage youth. It prohibits self-service sales techniques like cannabis vending machines, and restricts the use of marketing that would entice underaged youth to purchase cannabis.
The act also is working to develop controls for the safe production and sale of cannabis derived edibles, extracts, and topicals, with regulatory clarifications to come in October 2019. Ultimately the Cannabis Act aims to regulate quality control for cannabis production and to regulate the cannabis supply chain in order to create safe and legal cannabis products.
Cannabis growers who are cultivating the plant for sale need to be authorized by Health Canada and may need a license from the Canadian Revenue Agency to sell cannabis with an excise stamp to indicate it was produced legally. Some specific cannabis laws differ between the territories, but overall the Cannabis Act regulates the practices of Licenced Producers (LPs).
GPP requires LPs to ensure cleanliness of their facility and equipment. It also requires LPs to have a quality assurance program with a dedicated quality assurance person who is not linked to the production process.
Waves of Change
At this time Canada is working to harmonize its previous regulatory structure for medical marijuana with rules for producing both medicinal and recreational marijuana. Prior to the Cannabis Act, LPs producing medical marijuana were adhering to the requirements of the Access to Cannabis for Medical Purposes Regulations (ACMPR) which required adherence to Good Production Practices (GPP).
Quality Assurance Personnel
GPP requires LPs to ensure cleanliness of their facility and equipment. It requires LPs to have a
quality assurance program with a dedicated quality assurance person who is not linked to the
production process. These are also important requirements of Good Manufacturing Processed
(GMP).
An unbiased quality assurance person helps a business have checks and balances. The quality assurance (QA) person places products on hold until safety and quality testing is complete, then they interpret test results to determine if the product can be released for sale. This is very important because the QA person has the authority to make decisions that may not be in the best financial
interests of the cannabis producer, but is in the best interests of the consumer and in the best
compliance with regulations.
Good Production Practices and Good Manufacturing Processes
The GPP regulations are in the process of being archived and replaced with updated
regulations, but the original guidelines are still available online. While new regulations are not yet in effect, producers can still look to the GPP guidelines to ensure they are meeting the minimum requirements.
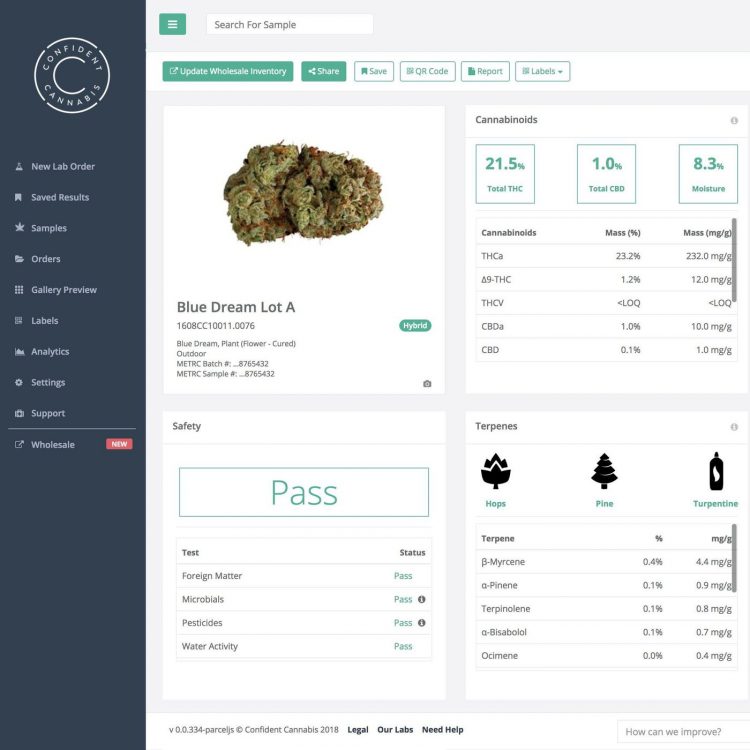
GPP requires testing for the following:
- Microbial contaminants of fresh cannabis, dried cannabis, and cannabis extracts
- Chemical contaminants like heavy metals, pesticides, and solvent residues for extracts
- Potency testing for delta-9-tetrahydrocannabinol, delta-9-tetrahydrocannabinolic acid, cannabidiol and cannabidiolic acid
These testing requirements are also found in GMP. While they have not yet been outlined in the new Cannabis Act, they are likely to remain the same. If the Cannabis Act employs more tenets of GMP production, or if producers voluntarily adopt GMP practices, additional testing and validation of testing will be needed.
Some additional testing for GMP include:
- Validations of test methods and operating procedures
- Environmental monitoring for chemical, microbial, and biological contaminants in the facility and on the equipment
- Testing of packaging and other materials that come in contact with the product
- Bioburden testing and validation to establish safe limits of microbial presence
Adapting GMP for the Cannabis Industry
Good Manufacturing Processes are a set of guidelines that are applied to the manufacture of most consumable products. GMP is an almost ubiquitous guidance in the US and had addendums specific to different product categories like food, cosmetics, nutritional supplements, medical devices, pharmaceuticals, and tissue products.
Because of the federal prohibition of cannabis in the US, the FDA has not made a specific addendum for GMP cannabis production. Instead, it has ruled (contrary to more than half of the US states) that regulation of cannabis falls under hefty and expensive pharmaceutical regulations that cannot be met by producers who are not multi-million dollar companies. Their justification for this claim, and re-assertion of cannabis as a schedule I drug, was the approval of the cannabis-based drug Epidiolex, and other cannabis-based pharmaceuticals which are in the process of clinical trials.
Canada is leading the way in national cannabis legalization with expert advice that guides licensed producers in how to apply existing GPP guidance to their cannabis businesses, and how to adapt GMP to the cannabis industry.
GMP identifies key points in production and creates a system that proactively controls production so the product is consistent and safe. GMP starts with a risk assessment and hazard analysis and critical control points (HACCP) plan, along with a quality manual that interprets the laws and defines them within the scope of the business. GMP goes beyond GPP production practices and creates a quality system from seed to consumer that covers all aspects of production.
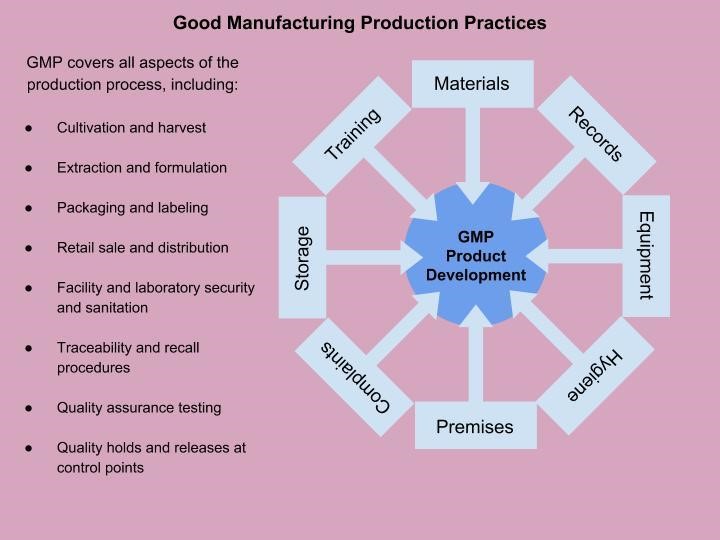
Designing Procedures to Meet GPP and GMP
If you already have GPP in place, you should have standard operating procedures, lab testing
procedures, security procedures, and a sanitation program. To bring your practices up to GMP
standards you will likely need to do further validation testing. Check out our post on GPP vs GMP to get a better understanding of this.
Validation Testing
Validations are tests that ensure processes reduce risks without introducing new risks. They often require further testing for microbial and chemical contaminants. One validation is usually not sufficient for a given practice. For example, an autoclave used to sterilize materials is usually validated annually, with monthly conformational testing to make sure the machine is reaching the right temperatures to sterilize even resistant microorganisms.
Sanitation Programs and Environmental Monitoring
In addition to having a sanitation program, GMP would require an environmental monitoring program. An environmental monitoring program first assesses the bioburden in a facility, that is how many microorganisms and what types of microorganisms are present through the production and storage areas. An environmental monitoring program is validated with initial monitoring results that are used to set alerts if levels of microorganisms are too high.
The sanitation program is validated through and with the environmental monitoring program. That is, the sanitation program uses the bioburden information to ensure sanitation practices target the amount and type of microorganisms present in the facility. An example of this would be, if molds are common to a part of the facility like the grow room, that room would be sanitized with a sporicide that targets mold between each crop.
In addition to having a sanitation program, GMP would require an environmental monitoring program.
GMP Facility Design
In order to have good sanitation practices, facilities should be designed in a way that allows for areas to be shut down for sanitation between crops. For many producers this means having multiple grow rooms and processing rooms so production can continue while other rooms are sanitized and tested to make sure sanitation was successful.
To make sanitation more successful and less cumbersome, building materials also need to be considered. Production areas and materials need to be made of nonporous materials that will not harbour moisture or contaminants.
GMP also requires that the facility be clearly divided into secured areas, areas where protective clothing is required, areas where the product is in-process, in quality hold, and an area where product has been approved and released from quality hold. This can be as simple as taping off and labelling containers, storage shelves, and threshold lines, or as difficult as requiring badge or code access to only certain employees who are authorized to enter particular areas.
GMP Documentation and Record Keeping
Traceability and documentation are another cornerstone of GMP. In order to have a sufficient system for recalling a product, all points of production need to be documented and records need to be kept so each batch of product can be traced through each step of production.
This means verifying material vendors, retaining samples of product and packaging, logging incoming materials, logging all steps of production, retaining laboratory test results, and having a quality assurance person sign off on all of these steps to ensure they are being met.
This creates an astronomical amount of paperwork when done by hand. In order to reduce that
paperwork, which can be easily misplaced, many producers are turning to quality management
software like GrowerIQ. These systems can go beyond keeping track of the product's journey from seed to sale, they can also integrate with digital environmental monitoring devices that log data with less need for staff to record things like daily grow room temperature and humidity
conditions.
GMP best practices means verifying material vendors, retaining samples of product and packaging, logging incoming materials, logging all steps of production, retaining laboratory test results, and having a quality assurance person sign off on all of these steps to ensure they are being met. This creates an astronomical amount of paperwork.
Choosing the "High" Road
As we move into 2019, Health Canada will be hard at work to establish guidance for industry
that will likely bridge GPP and GMP principles. If you are ready to be an early adopter instead of playing regulatory catch up, you should start now by working with a consultant, or an experienced Master Grower, that will help you interpret regulations and transform them into proactive practices.
For more information, and actionable tips from our Master Grower on how to improve your yields and processes, please click below to learn more about our GPP and GMP cannabis consulting services.
About GrowerIQ
GrowerIQ is changing the way producers use software - transforming a regulatory requirement into a robust platform to learn, analyze, and improve performance.
To find out more about GrowerIQ and how we can help, fill out the form to the right, start a chat, or contact us.

